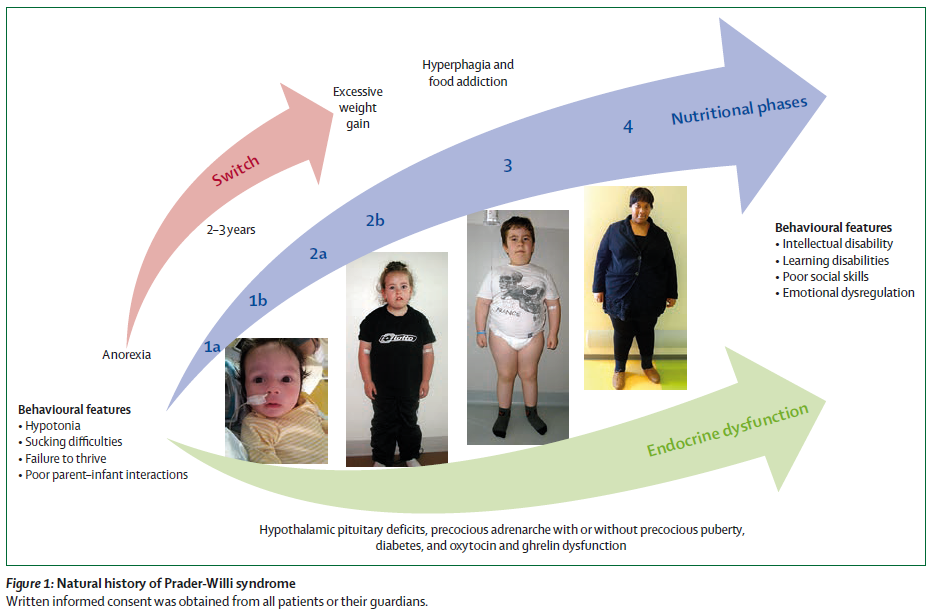
Pétition (en anglais) – à signer avant le 23 mai – DCCR de SOLENO Therapeutics
L’association Prader-WIlli US (PWSA US) a lancé une pétition pour soutenir la démarche accélérée qu’a obtenu SOLENO pour son futur médicament DCCR en lien avec l’hyperphagie et le syndrome de Prader-Willi.
Si vous en avez la possibilité, n’hésitez pas à signer la pétition!
voici le lien : https://www.surveymonkey.com/r/X3WCGDT
Big news! Soleno Therapeutics has announced a groundbreaking achievement: diazoxide choline (DCCR) has been granted Breakthrough Therapy Designation by the FDA for Prader-Willi syndrome (PWS). This marks a significant milestone as the FIRST-EVER designation for a drug developed for PWS. The designation underscores the FDA’s recognition of PWS as a serious condition and the potential for DCCR to offer substantial improvement for patients with hyperphagia. While this is a major step forward, it’s important to note that DCCR’s approval is still pending. However, Soleno plans to submit its New Drug Application in mid-2024. To help ensure the FDA pays attention to this NDA, we encourage our community to sign the petition by May 23rd here: https://lnkd.in/gq2tMaFn
Read Soleno’s full press release announcement at https://lnkd.in/gGDwTS97 or